Soon enough, flyaways start to form and all your hard work is undone thanks to the ultimate environmental villain—frizz.
But as with every good villain, frizz is just misunderstood. Let’s dive into the villain origin story, i.e. where it comes from + why it happens, what it does to hair at the molecular level, and whether it can truly be conquered.
what causes frizz?
A common misconception is that humidity is the only thing to blame for frizz. Frizz can also occur in cool, dry environments. But before we talk about environmental factors, we have to address the unknown elephant in the frizz room—one of the root causes of frizz is damage to hair.
So all that stuff your clients do to express themselves through their hair—think chemical services like coloring and bleaching, environmental toxins, sun exposure, and stress from heat styling and brushing—all that is also leading to more frizz.
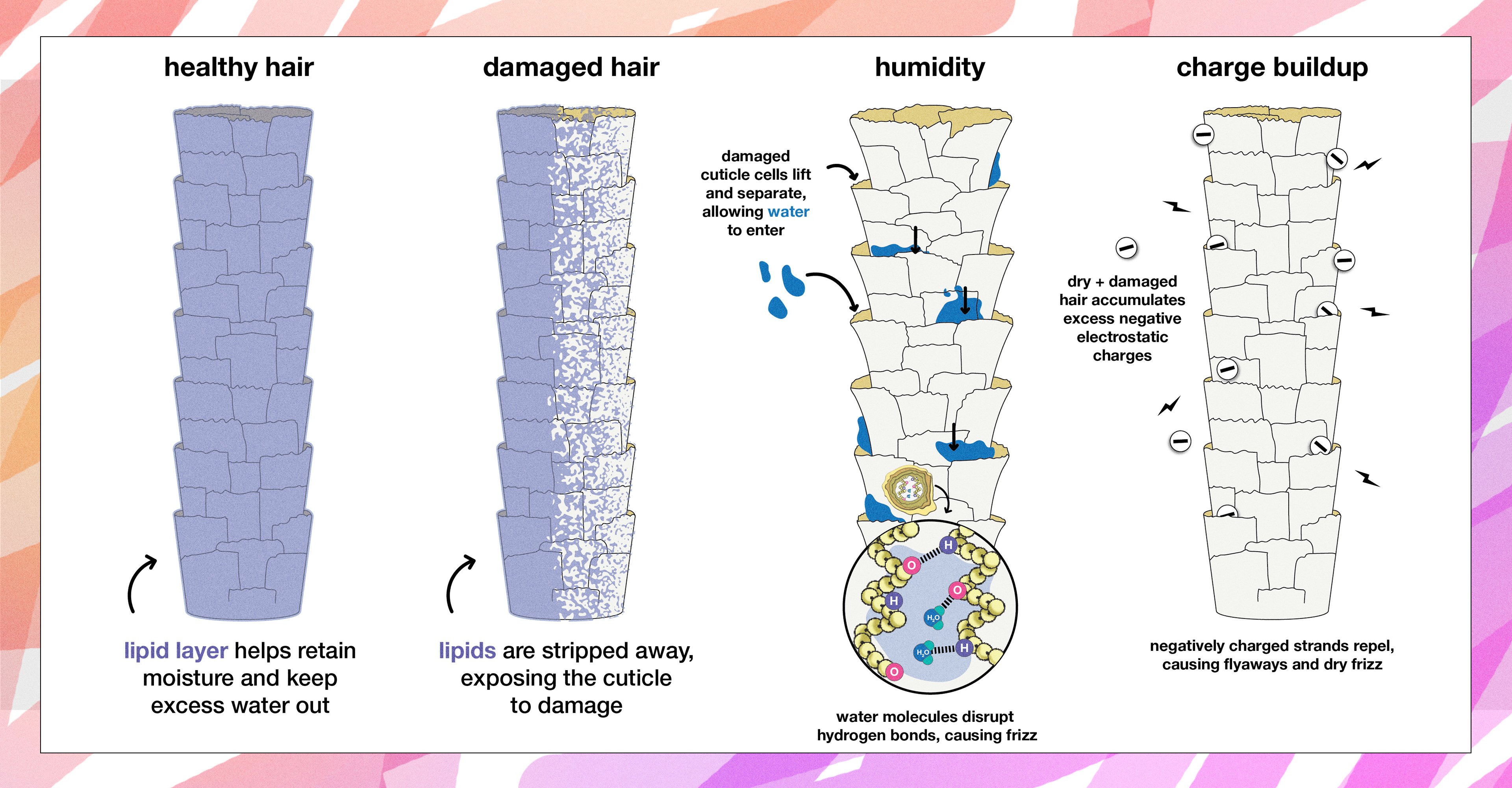
why does damage to hair cause frizz?
Hair’s structure is made up of different layers (explore the layers with our AR experience). An additional lipid or natural oil layer on the surface of hair helps it retain moisture and keep excess water out.
When the lipids on the surface of hair are stripped away through damage, the cuticle layer underneath can be damaged more easily. The cuticle is composed of overlapping shingle-like cells that protect hair from the outside world. When healthy, these cuticle cells lay flat. But when hair is exposed to damage, the overlapping cells lift and separate.
Separation at the cuticle level makes it easier for water to pass through the cuticle and hydrogen bond with the keratin chains in hair. This increases the likelihood of frizz.
Damage also makes the internal proteins in hair more negatively charged. That means that hair can more easily attract water, and strands become more prone to repulsion, contributing to frizz.
say more things about bonds…
The shape and strength of hair is determined by various chemical bonds. But one type of bond—hydrogen bonds—is at the origin of frizz.
Hydrogen bonds are bonds that help hair maintain its shape. In a “no-frizz” state, the amino acid groups in the interwoven polypeptide chains that make up hair’s structure are hydrogen bonded to each other to support a temporary shape or style.
But water is REALLY good at hydrogen bonding. When water from the air is introduced into the hair, it hydrogen bonds with the amino acid groups that give hair its structure. Water disrupts hydrogen bonds between neighboring keratin chains, causing hair to change shape. The shape of hair resets as the water evaporates and the hydrogen bonds between keratin chains are restored.
Another culprit for frizz is charge buildup. Hair naturally carries a negative charge. However, dry and damaged hair accumulates excess negative electrostatic charges, that are caused by a buildup of negatively charged particles called electrons. Since like charges repel, strands try to stray as far away from each other as possible, causing flyaways and dry frizz or static hair.
do anti-frizz products really work?
When you walk down the haircare aisles of your favorite beauty supply store, you see plenty of products that claim to tame frizz. Most of these products are oils and cationic polymers.
Oils provide a temporary layer of protection that slows water from passing through the cuticle and causing frizz—these sort of serve as a stand-in lipid layer. While some oils can penetrate past the cuticle and provide deeper protection, many sit on the surface and are quickly stripped away by shampoo during your client’s next wash. To combat frizz, you must reinstall their hair’s lipid barrier protection at multiple points—not just temporarily on the outermost layer.
Cationic polymers are positively charged polymers that are attracted to negative charges and temporarily neutralize the charge buildup on the surface of hair. By neutralizing charges, they reduce flyaways and frizz. But since they readily dissolve in water, they are easily washed away. So, like oils, cationic polymers are a band-aid solution for the real problem: damage.

so, what should I do to fight frizz?
The main cause of frizz is cuticle damage, but most anti-frizz products focus on temporarily coating the surface of hair instead of repairing the deeper damage. The true key to stopping frizz in its tracks is to take a holistic approach to hair care.
No hair issue exists in a vacuum—hair is a complex structure with many different compounds, layers, and chemical bonds that work in harmony to give hair its strength, elasticity, and shape. So, instead of focusing on short-term styling outcomes, focus on a holistic hair routine that prioritizes molecular repair from the inside out. That’s the real secret to long-term hair health—and taming frizz.

